25. How correctly do I've to prepare TOC standard and system suitability Answer concentrations for screening Bulk Water?
Professor Steve Chadban from Royal Prince Alfred says approximately twenty five litres of water for each minute is shipped down the drain when water is remaining purified for dialysis devices.
Documentation verification is often a test in which the status needs to be checked according to the project agenda around the IQ specifically, normally the IQ examination may very well be open up until eventually both equally IQ and OQ are All set and the final documentation continues to be copied.
Having said that, it could occasionally be suitable to execute it along side OQ or method validation.
The general performance is According to the general performance requirements laid out in the URS (this exercise is named PQ).
This really should be the exact same excellent of water as Employed in the relevant producing stage (the Preliminary rinse is often purified water)
two. It can be total documented verification with the system that it works all through the method According to functioning ranges continually.
The doc numbering system for miscellaneous get more info validation study shall be maintained According to Annexure- four
It really is For that reason that endotoxin control of water systems is of paramount relevance in relation for the manufacture of sterile medicines, In particular those that are administered intravenously. To get a pyrogenic response to be triggered, there ought to be substantial portions of endotoxin in the blood stream (endotoxemia), derived from substantial figures of Gram-damaging germs.
four. Any major modify in the process equipment or any maintenance work performed right after any major breakdown
This grade of water is also equipped for cleaning of item Call gear and elements, and it's the water source to autoclaves in the form of steam. Purified water is utilized for products processing; it really is equipped to laundries, utilized for hand washing, and as the source water for distillation.
ANSBACH, Germany — German engineers switched on the water treatment method system at U.S. Army Garrison Ansbach this 7 days to begin the very long-planned purge of toxic PFAS chemical substances from groundwater exiting read more The bottom.
Plan monitoring, servicing, and alter Command procedures also are necessary to be certain ongoing system operation and water good quality as specified.
Along with these Key methods, other purification actions for example deionization, carbon filtration, and UV disinfection will often be A part of WFI systems to make certain the very best good quality of water.
 Alana "Honey Boo Boo" Thompson Then & Now!
Alana "Honey Boo Boo" Thompson Then & Now! Lark Voorhies Then & Now!
Lark Voorhies Then & Now! Michael Bower Then & Now!
Michael Bower Then & Now! Mike Vitar Then & Now!
Mike Vitar Then & Now!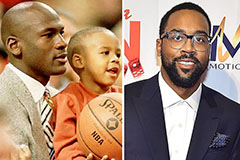 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!